Another Flipping Organic Course, part 2
by Vince Maloney
Questions?
As mentioned in the first post, this blog chronicles something of a personal journey as I “flipped” my organic course. For each post, the plan is to cover a specific topic. Since they were areas that I identified as needing improvement and are of greatest concern for the continued use of flipping, I’ll start with topics where something could have been done better. So, this post will consider the questions used in the face to face class.
At the most recent BCCE this past August, there were around 40 talks about flipping courses in every area of chemistry with an entire symposium devoted to organic chemistry alone. An interesting picture of flipping developed as one talk followed another. Although there were many similarities in the courses as to what to lead to success with the students, no two speakers had quite the same experience. For example, where many found it necessary to provide varying amounts of review before proceeding to the in class questions, a few, such as myself found that their students preferred getting right down to business and didn’t want a review. Other the hand, I was with the majority in providing short 5 – 10 minute online lectures prior to class while others stayed with a 50 or 90 minute format for their online lectures. Although I refer to this blog as a personal journey, perhaps you will find that much of what is written here resonates with your attempt to flip a portion or all of your class. In other cases, you may need to do something completely different. Those with more experience and depth of knowledge related to flipping their courses, are certainly welcome to comment and provide your insights.
Besides recording all of the lectures for the two semester sequence, in class activities had to be provided for each and every session. For years, my “traditional” lecture was laced with classroom assessment technique (CAT’s) questions. Also classes were followed by twice a week review sessions where many homework problems were covered. While considering the “in class” questions, it became apparent that the majority of the CAT’s questions would be unsuitable for use. Meanwhile it appeared to be a simple task to take all of the previous review session questions and modify them for the class. This assumed the model of time shifting where the lectures are moved outside of the face to face class while the homework problems are moved into it. Since CAT’s questions are often meant to check comprehension of a lecture topic that has just been covered and often are rather simple, it made sense and turned out to be the case that many would not belong in the face to face class. Some were retained in the online lectures where the students were asked to momentarily stop the lecture and consider a question that tested their understanding of a topic. The surprise came when it turned out that moving the review session questions into the face to face to class did not work as smoothly as expected.
Over time it became clear that asking the questions in a semi-random order that worked so well for the review sessions didn’t seem to be as beneficial in the face to face class. Instead, it was better to ask questions that were roughly following the topic order in class and to use “scaffolding.” This latter technique refers to building up the question complexity until the class is functioning at the desired level of rigor. Scaffolding can also refer to writing the questions in a manner that shows the students how to approach the problem. Electrophilic addition of HX along with acid catalyzed hydration and NMR spectroscopy can be used as examples.
For electrophilic addition and acid catalyzed hydration, the following sequence of question topics could be asked. (The topic order and scaffolding choices were based on using Jones and Fleming for a text.)
- Product of the addition of HX to a symmetrical alkene
- Mechanism of addition
- HOMO and LUMO in each step
- Acid catalyzed hydration of a symmetrical alkene
- Mechanism of acid catalyzed hydration
- Role of the acid catalyst
- Addition of HX to an unsymmetrical alkene: 2-methylpropene
- Regiochemistry and Markovnikov's rule
- Carbocation stability
- Inductive effects, hyperconjugation, polarizability and alkyl groups
- Resonance effects and addition to vinyl halides and vinyl ethers
- Stereochemistry of addition
- Formation of both enantiomers
- What happens if there is no good nucleophile
- Carbocationic polymerization
- Lewis acids and initiation
- Suitable alkenes
- Carbocation rearrangements and addition
- Preference for more substituted carbocation
- Carbocation rearrangements and ring expansion and contraction
For NMR, the following topic order for questions could be used.
- Assignment of chemical shifts in 1H NMR spectrum of p-methylbenzaldehyde
- Propyl propanoate spectrum: assignment of provided structural fragments based on chemical shift and splitting patterns (total structure not provided)
- Determination that propyl propanoate structure fits the spectrum in the previous question best
- Students propose structure of compound of dipropyl ether based on molecular formula, chemical shifts, splitting, and integration in its 1H NMR spectrum. (Degrees of unsaturation covered earlier with IR)
- Protons on N and O in NMR
- The appearance of two doublets in the 1H NMR spectra of p-substituted aromatic rings
- Predict the multiplicity of protons in various structures
- 13C NMR chemical shifts and p-methylbenzaldehyde
- Predict the number of peaks in a 13C NMR spectrum of p-methylbenzaldehyde
- The students were given the molecular formula, IR, 1H NMR and 13 NMR spectra of the following compounds in order and asked to propose structures. The spectra came from A Spectrum of Spectral Problems by Richard A. Tomasi
- 2,4-dimethylpentan-3-one
- p-ethoxybenzaldehyde
- p-ethylacetophenone
- isooctane
- benzyl butanoate
- 2-ethoxyethyl acetate
- Homotopic, enantiotopic, and diastereotopic protons
Most of the questions were of the type found in most organic texts except that they were adapted for use with classroom response systems. Eventually, I plan to post the questions that have been used during the course of the year. However, questions that are from copyrighted material must be culled first. They could be used in the class under fair use but should not be posted on OrganicERs.
Another area that needed improvement was the amount of time spent on some topics. My tendency was to add a few too many questions. I fell into the trap of thinking that if I just added 1 or 2 more questions, they would really understand the topic. This caused me to go too slow on some topics and too fast in others. The course pace was uneven. Next time, it will be necessary to be more judicious in choosing questions and leave some questions as some type of post class homework. Recently, I was able to do this more successfully in a general education chemistry course that I taught this fall. In a future post, I’ll cover pre- and post-class assignments and their use.
Since the class size was approximately 100 students, the iClicker classroom response system was used to poll the students for each in class question. This meant that although more sophisticated questions were asked, the format was limited to the capabilities of the iClicker2 and iClickergo phone app. However, this allowed for more than multiple choices questions. Both text and numerical questions could be asked. For example, students could be asked to text in the name of a functional group projected on the screen. For a numerical response, they could be asked to enter the number of peaks that would be observed in the 13C NMR spectrum of benzophenone. Numerical responses can be used to ask both mechanistic and synthetic questions. Two examples follow. The answers that the students should have entered are given as numbers in blue.
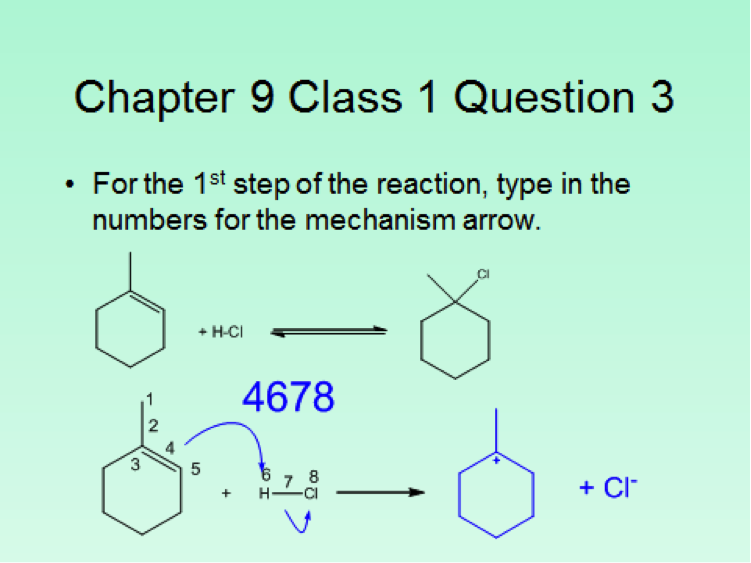
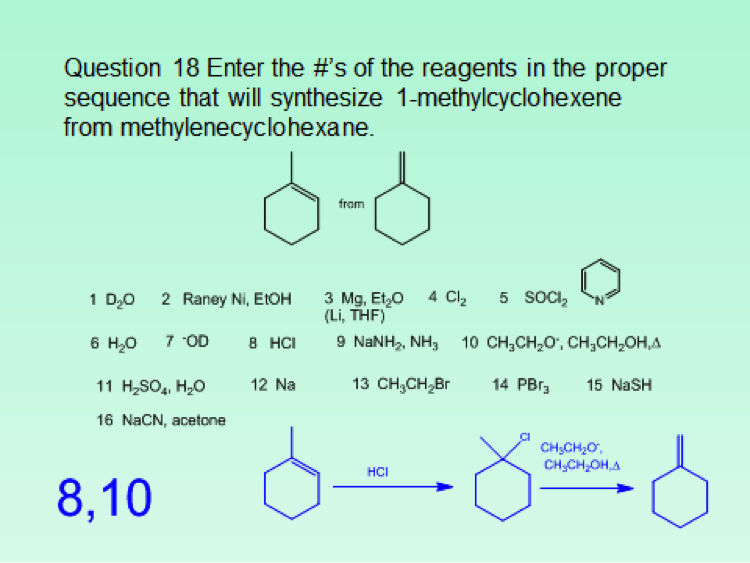
Another option for multiple choice questions is to go around the room and solicit responses from the students, place 5 different answers on the board, and then let them vote on what they consider to be the best answer. This worked particularly well for spectroscopy questions. Finally, although it’s more of a guide for classroom assessment technique type questions, Suzanne Ruder’s book Clickers in Action: Active Learning in Organic Chemistry is a useful guide for constructing clicker questions.
